Have you ever wondered how shining sunlight on a solar panel creates electricity?
It is, unsurprisingly, quite a complex operation. It’s called the photovoltaic (PV) effect, and in this article I will try and explain it as simply as I can.
An atom consists of protons (positive charge), neutrons (neutral charge), and electrons (negative charge). Electron movement – that is, electrons moving from one atom to another (DC), or moving back and forth (AC) – is what we call electricity.
You start with atoms of silicon, a metalloid (meaning it has properties between that of a metal and non-metal). The biggest producer of silicon is China, followed by Russia and then the USA. Solar panels are usually made of silicon, and it’s this element that does all the hard work. However, silicon by itself is not a very good conductor of electricity.
However, you can “dope” silicon with other atoms, such as boron or phosphorous. Doping is adding a small number of atoms to a large number of another – for example, 1 boron atom in 10 silicon atoms. In actuality, it’s even less than that – perhaps one in a trillion.
Adding one boron atom to a group of silicon atoms creates one “hole” – a space where an electron would be in pure silicon, but isn’t. This is called p-type doping (p is for positive – as there is one fewer electron than in a normal “neutral” state, this makes the resulting mix slightly positive).
Adding one phosphorous atom to a group of silicon atoms adds one extra electron. This is called n-type doping (n for negative, for opposite reasons to p-type).
The magic happens when you bring some n-type material next to some p-type material, and create what’s called a p-n junction. Some of the extra electrons in the n-type move to the holes in the p-type, recombine, and create what’s called a depletion region (also known as the charge space region) between the n-type and p-type. Once this depletion region reaches a certain size (depending on the doping concentration), no more electrons can flow across the border, as an electric field is induced across the junction.
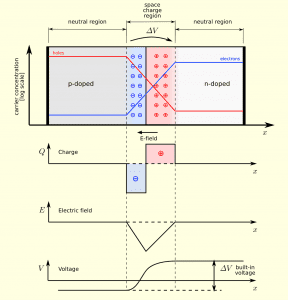
Image source: https://commons.wikimedia.org/wiki/File:Pn-junction-equilibrium-graphs.png
Bring in the sun. As rays of sun (called photons) enter the p-n junction (especially in the depletion zone), the solar energy (which we normally feel as heat) is absorbed. This gives some of the electrons enough energy to “break free”, and creates a new electron-hole pair – that is, a free electron, and an electrically-charged space where an electron should be.
Now, remember one side of the depletion zone has extra holes (p-type) and one side has extra electrons (n-type)? Well, this photon-generated electron (negative charge) is attracted to the holes in the p-type side (positive charge), and the photon-generated hole (positive charge) is attracted to the electrons in the n-type side (negative charge). If the p-type and n-type sides are connected through a load – that is, an external circuit, which could include your toaster or TV – the photon-generated electrons and holes can leave the p-n junction and travel towards each other through the wires – and through the toaster and the TV – and eventually recombine. This flow of electrons (and holes) is the electricity generated by solar PV – flowing through the toaster and TV is what makes them work!
As an aside, this is also how LEDs work – but in reverse! If you apply current/voltage to a p-n junction, the extra energy escapes as light.










